Understanding the Role of Literature Review in Clinical Study Design and Execution
Understanding the Role of Literature Review in Clinical Study Design and Execution
- Home
- Academy
- Research-Methodology
- Role of Literature Review in Clinical Study
Role of Literature Review in Clinical Study
- 2. Identifying Gaps and Guiding Research Design
- 3. Enhancing Study Rigour and Reducing Bias
- 4. Evidence Synthesis: Systematic Reviews and Meta-Analyses
- 5. The Role of Quality Assessment in Literature Reviews
- 6. Informing Evidence-Based Practice
- 7. Support the development of clinical documents
- 8. Challenges in Conducting Literature Reviews
- Conclusion
- Reference

Recent Post
Understanding the Role of Literature Review in Clinical Study Design and Execution
1. Defining the Literature Review in Clinical Research
A literature review is a methodical review and synthesis of the available evidence on a certain topic, providing a thorough overview of what we already have knowledge of and what needs to be further explored (Munn et al., 2018). In clinical research, such reviews help the researcher gain knowledge of the current state of the topic in terms of knowledge development, effectiveness of intervention, and data analysis for future research. The review process often entails systematic reviews and meta-analysis, which are especially helpful in clinical research to summarise a mass of evidence (Wilt & Fink, 2021).
2. Identifying Gaps and Guiding Research Design
The vital role of a literature review in clinical research is to identify gaps in the current knowledge base. By reviewing existing literature, researchers can identify areas where there is a lack of evidence or inconclusive evidence. This informs the researchers’ ability to develop relevant research questions and hypotheses, thereby guiding the research design. A systematic review, for example, assists in determining whether an intervention’s effects have been studied among different populations or in different clinical contexts; a systematic review will provide the information the researchers require to more directly narrow their research (Mathew, 2022).
The review stage is critical for identifying the best research design in clinical research. For example, a review of the literature may show that RCT design has been used effectively in prior studies to test the effectiveness of a drug, which suggests to the researcher that they should use that same design (Page et al., 2021).
3. Enhancing Study Rigour and Reducing Bias
Reviews of the literature are key to increasing rigour in clinical studies. When researchers can critically review previous studies, they actually review the quality and relevance of these studies to their own project. This is especially important when distinguishing between potential sources of bias and variability that could influence outcomes in the present study. For example, systematic reviews of diagnostic accuracy studies can provide helpful information regarding some types of methodological issues researchers can address (Whiting et al., 2004).
In addition, literature reviews can help in minimising bias in clinical studies by providing a balanced perspective of the evidence. As an outcome of synthesising studies utilising different methodologies with the quantitative and qualitative studies of evidence, researchers account for variance in perspectives, making findings more reliable and validated. This is particularly true when considering outcomes of complex studies in clinical studies regarding different features of the intervention, with participants having study-specific factors that may not apply to a separate study (Noyes et al., 2019).
4. Evidence Synthesis: Systematic Reviews and Meta-Analyses
Systematic reviews and meta-analyses are core methodologies used in the literature review component of clinical research. They offer a structured and transparent approach to summarising the evidence, allowing more practical conclusions to be drawn. A systematic review follows a pre-determined protocol to find, assess, and summarise all relevant studies on a given topic. A meta-analysis is a study that selects multiple studies and employs statistical methods to calculate an overall effect size (Vetter, 2019). Both systematic reviews and meta-analyses are methods that aggregate the evidence from multiple studies and help determine whether their findings can be generalised to new patients.
5. The Role of Quality Assessment in Literature Reviews
The calibre of studies in a literature review is a key determinant of the implications of clinical research. Therefore, it is important that researchers assess the methodological quality of studies to achieve synthesizing evidence that is trustworthy and has validity. Common quality assessment tools include the Newcastle-Ottawa Scale (NOS) for observational studies and the Cochrane Risk of Bias Tool (ROB-2) for randomized trials (Shea et al., 2017; Sterne et al., 2016). These tools determine the presence of biases that may jeopardize the effectiveness and assess that only high-quality studies are being used for review.
In addition to assessing quality, it is also important for researchers to assess the heterogeneity of studies included in systematic reviews. For instance, differences in designs, size samples, or measures of outcomes will impact the conclusions formed from the findings of the systematic review. Researchers should assess and account for heterogeneity using statistical measures, such as subgroup and/or sensitivity analyses, in order to account for the differences, so that an evidence’s claims are grounded in confidence.
6. Informing Evidence-Based Practice
One of the main purposes of literature reviews in clinical research is to promote evidence-based practice. Literature reviews synthesize the best available evidence and provide it to clinicians to make informed decisions regarding patient care. Sataloff et al. (2021) stated that systematic reviews are highly useful for developing clinical practice guidelines to best reflect current and high-quality evidence.
As an example, a literature review of the effects of a certain drug in the treatment of a disease can inform health care providers of the drug’s effectiveness, safety, and side effects. The results from the review can then be used to develop clinical practice guidelines so that patients are given the best treatments available, based on current research evidence. Evidence-based practice is extremely important, as it can improve patient outcomes while minimising the risk of harm to patients.
7. Support the development of clinical documents
Literature review assists regulatory medical writers in developing the clinical documents, where they need to mention the treatment gap in existing drugs or medical devices. This describes the need for a new drug that is efficient and produces no or fewer adverse effects than the existing drugs. For specific diseases, some classes of medication may not be efficient or produce severe side effects.
Example:
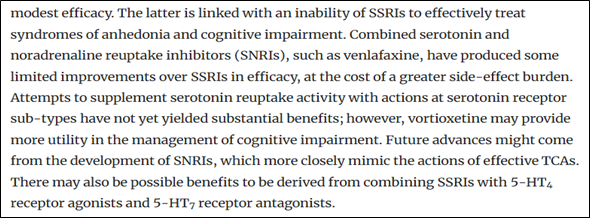
Figure 1 illustrates the inefficiency of SSRI to treat syndromes of anhedonia and cognitive impairment (Source: Cowen, 2023)
Below is the model of Common Technical Document module 2.5, written based on the gap in the existing treatment class (SSRI) from the above literature.
8. Challenges in Conducting Literature Reviews
Although they are fundamental to the knowledge production process, literature reviews in clinical research are not easy to conduct. One problem researchers may have is to carry out a comprehensive and accurate search strategy with multiple publications; then you must complete a literature review. To enhance the completeness of the literature reviewed, Cooke et al. (2012) propose to extend the literature reviews beyond the classic PICO (Population, Intervention, Comparison, Outcome), which may enable a more comprehensive literature review and understanding of the research question.
Another difficulty is including grey or unpublished literature in systematic reviews, which is paramount in the avoidance of type 1 error.
Conclusion
To conclude, literature reviews are the backbone of clinical research in the research process. They provide a basis for evidence-based, high-quality research studies. They also allow researchers to fill a gap in knowledge, guide research design, and assist in ensuring methodological rigour while impacting evidence-based clinical practice. Systematic reviews, meta-analyses, and quality assessments comprise a thorough literature review at the clinical level of research. However, there are complexities involved in the literature review process, but a well-done literature review will increase the quality and credibility of clinical research and ultimately aid patients and practitioners in clinical care.
Ready to find a research gap for your clinical study?
Our team of PhD experts can offer you tailored assistance in designing precise research questions and selecting suitable methods, while ensuring compliance with scientific and ethical standards.
PhD Assistance will help you at all levels and in all ways with any research writing you need. Contact PhD Assistance right away.
References
- Munn, Z., Stern, C., Aromataris, E., Lockwood, C., & Jordan, Z. (2018). What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol, 18, 1.
- Wilt, T. J., & Fink, H. A. (2021). Systematic reviews and meta-analyses. In Clinical Research Methods for Surgeons (pp. 311-325). Humana Press.
- Mathew, J. L. (2022). Systematic reviews and meta-analysis: A guide for beginners. Indian Pediatr, 59, 320-330.
- Page, M. J., Moher, D., Bossuyt, P. M., et al. (2021). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ, 372, n160.
- Whiting, P., Rutjes, A., Dinnes, J., Reitsma, J., Bossuyt, P., & Kleijnen, J. (2004). Development and validation of methods for assessing the quality of diagnostic accuracy studies. Health Technol Assess, 8(1).
- Noyes, J., Booth, A., Moore, G., Flemming, K., Tunçalp, O., & Shakibazadeh, E. (2019). Synthesising quantitative and qualitative evidence to inform guidelines on complex interventions: Clarifying the purposes, designs and outlining some methods. BMJ Glob Health, 4(Supplement 1), e000893.
- Vetter, T. R. (2019). Systematic review and meta-analysis: Sometimes bigger is indeed better. Anesth Analg, 128, 575-583.
- Shea, B. J., Reeves, B. C., Wells, G., et al. (2017). AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ, 358, j4008.
- Sterne, J. A. C., & Hernán, M. A. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ, 355, i4919.
- Sataloff, R. T., Bush, M. L., Chandra, R., et al. (2021). Systematic and other reviews: Criteria and complexities. J Neurol Surg B Skull Base, 82, 273-276.
- Cowen, P.J. (2023). SSRIs in the Treatment of Depression: A Pharmacological CUL-DE-SAC?. In: Browning, M., Cowen, P.J., Sharp, T. (eds) Emerging Neurobiology of Antidepressant Treatments. Current Topics in Behavioral Neurosciences, vol 66. Springer, Cham.
- Cooke, A., Smith, D., & Booth, A. (2012). Beyond PICO. Qual Health Res, 22, 1435-1443.

